MINDSET FOR INNOVATION
Whether you are in the initial development phase, need analytical and stability testing, or are still In the Research And Development stage, Our team is always ready to work together to deliver your ideal product.
We support our customers through robust innovations catering to the unique market needs. begining with need gap and product discovery.
RESEARCH
DEVELOP
FORMULATE
SCALE
DELIVER

The history of softgels in the pharmaceutical and dietary supplement sectors is rich. We provide high quality soft gelatin products with a strong track record of developing and launching complex formulations. When you work with us, you’ll also learn:
ABILITY TO CUSTOMISE TO REQUIREMENTS
CUSTOMERS COLLABORATE WITH OUR TEAM OF PROFESSIONALS
DEVELOP CONTEMPORARY ENCAPSULATED ITEMS
COMPLETELY CUSTOMISABLE
RESEARCH & DEVELOPMENT
Our highly skilled team of scientists and engineers in the R&D department are responsible for developing new products and improving existing ones.
In order to develop innovative products that enhance patient lives, our team works closely with our clients to keep abreast with the latest in consumer health requirements.
All products are developed in accordance with good manufacturing practices (GMPs) and are subject to rigorous testing before they are released to the market.
As a leading manufacturer of industry,SHPL strives to continuously improve its softgel production process to ensure that the products are of the highest quality.
Analytical Research and Development (AR&D):
AR&D plays an essential role in this process. It analyses the data from laboratory tests and other research to determine the best strategies and practices for our manufacturing.
The quality of the raw materials used, assessing the stability and shelf-life of the finished product, and ensuring that the product is safe and efficacious for use..
As a leading manufacturer of industry,SHPL strives to continuously improve its softgel production process to ensure that the products are of the highest quality.
REGULATORY AFFAIRS
QUALITY CONTROL
Our quality control complies with National and International standards by strict adherence to cGMP, Quality Management System (QMS) and Good Laboratory Practices (GLP).
Work process is driven by a robust quality management system (QMS)
SECURE STORAGE AND COMPLETE DATA INTEGRITY
- State of the art analytical instruments with audit trail enabled and networked with FDA 21 CFR part 11
- Adopts established pharmacopoeia methods such as USP, BP, IP & others
TEAM OF DEDICATED SKILLED AND EXPERIENCED SUBJECT MATTER EXPERTS.
- Testing capability of Routine API & Excipient Analysis.
- Intermediate and Finished product analysis
- Packing material Analysis, Contaminant Analysis
- Microbial analysis (Bio-burden of RM/PM & Finished products, Environmental/Personnel monitoring)
- Rupture & Dissolution Analysis
- Physical Analysis
- Product Stability Evaluation
- Photo stability Evaluation
- In-house Working standard development
ACCREDITATION
GLOBAL PRESENCE
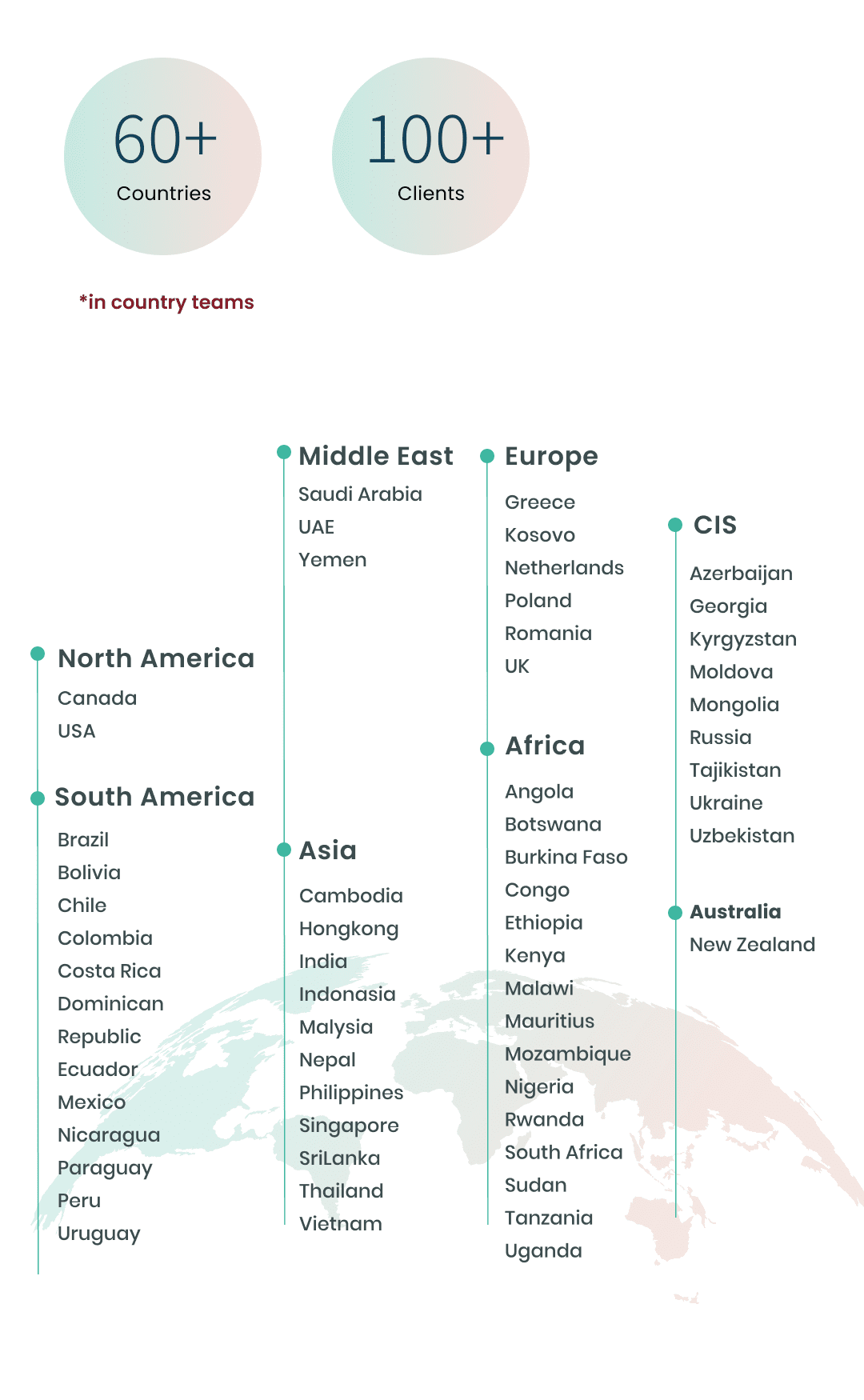
COUNTRIES
CLIENTS
North America -
- Canada
- USA
South America -
- Brazil
- Bolivia
- Chile
- Colombia
- Costa Rica
- Dominican Republic
- Ecuador
- Mexico
- Nicaragua
- Paraguay
- Peru
- Uruguay
Africa -
- Angola
- Botswana
- Burkina Faso
- Congo
- Ethiopia
- Kenya
- Malawi
- Mauritius
- Mozambique
- Nigeria
- Rwanda
- South Africa
- Sudan
- Tanzania
- Uganda
Europe -
- Greece
- Kosovo
- Netherlands
- Poland
- Romania
- UK
Middle East -
- Saudi Arabia
- UAE
- Yemen
Australia -
- New Zealand
Asia -
- Cambodia
- Hongkong
- India
- Indonasia
- Malaysia
- Nepal
- Philippines
- Singapore
- SriLanka
- Thailand
- Vietnam
CIS -
- Azerbaijan
- Georgia
- Kyrgyzstan
- Moldova
- Mongolia
- Russia
- Tajikistan
- Ukraine
- Uzbekistan